Hexagonal diamond is an allotrope with a similar structure to cubic diamond but superior properties. However, the formation conditions for hexagonal diamond are extremely demanding. The greatest challenge in artificial synthesis is that the formation energy of hexagonal diamond under high temperature and high pressure is higher than that of cubic diamond. Therefore, the product produced under high temperature and high pressure is usually cubic diamond, making hexagonal diamond difficult to obtain.
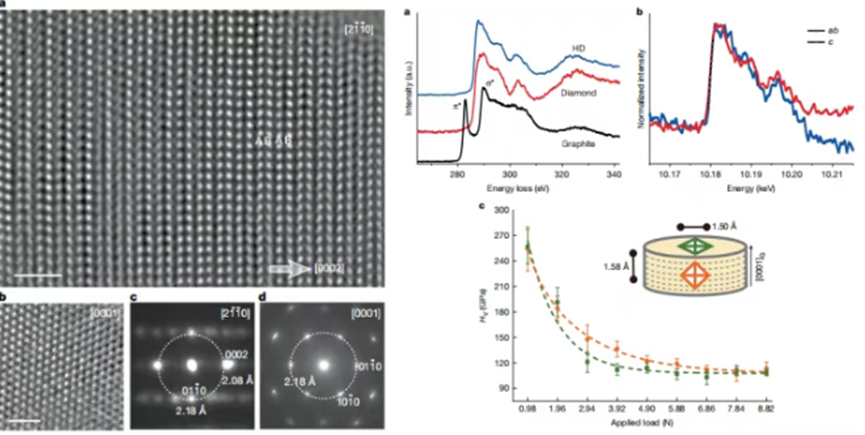
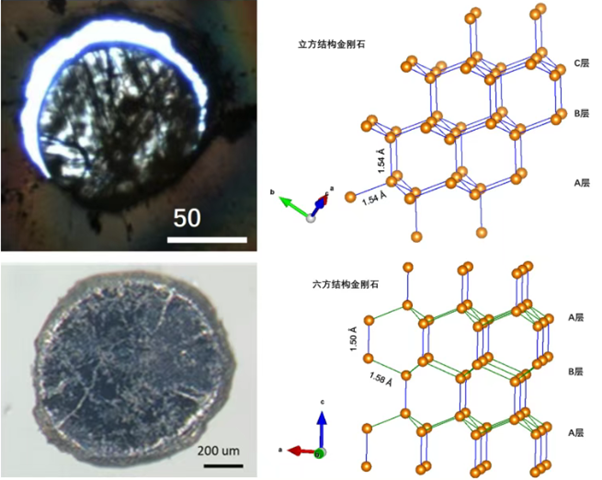
Chinese scientists have innovatively proposed a method for transforming graphite into hexagonal diamond. Using diamond anvil cell (DAC) technology, they successfully transformed high-quality graphite single crystal precursors into highly ordered hexagonal diamonds on the order of hundreds of microns under controlled high temperature and high pressure (quasi-hydrostatic pressure). Using in situ single-crystal X-ray diffraction, the team accurately revealed for the first time the crystallographic orientation relationship during the graphite-hexagonal diamond phase transition, systematically elucidated the phase transition mechanism, and confirmed the unique triple twinning structure of the synthesized sample. To accurately characterize the mechanical properties of hexagonal diamond, the team further developed a large-cavity press synthesis process. By optimizing the combination of solid pressure-transmitting media, the differential stress effect was effectively suppressed, ultimately achieving the successful production of millimeter-scale hexagonal diamond bulk samples under the extreme conditions of 20 GPa/2073 K.
The research's innovations include:
1. Using in situ single-crystal X-ray diffraction, the crystallographic pathway of the graphite-to-hexagonal diamond transition was revealed at the atomic scale for the first time, clarifying the phase transition mechanism.
2. Utilizing a combination of multi-scale characterization techniques, including high-resolution transmission electron microscopy (HRTEM), X-ray Raman spectroscopy, electron energy-loss spectroscopy (EELS), and UV Raman spectroscopy, the sp3 hybridization of hexagonal diamond was confirmed, demonstrating that it shares the same chemical bonding mechanism as cubic diamond, but exhibits double bond length characteristics.
3. Vickers hardness tests show that hexagonal diamond exhibits mechanical properties comparable to cubic diamond, providing direct evidence for its application in superhard materials.
This systematic study not only resolves over 60 years of controversy regarding the macroscopic existence of hexagonal diamond but also lays a solid foundation for the development of hexagonal diamond as a next-generation high-performance functional material.